
GMP Qualification
Machines and Plant Qualification
In the medical technology and pharmaceutical industry, the execution of a system qualification is an elementary part of the quality management system and a prerequisite for obtaining and maintaining the manufacturing permit
The qualification is an integral part of the GMP requirements and an essential milestone for further validation work. It is the documented evidence that a technical system is properly designed and installed according to the customer’s requirements and that it works exactly as it was originally designed by the plant/ machine operator.
A qualification also proves that the products produced permanently meet the regulations and standards – also they are GMP compliant. The specific requirements for qualification and validation are defined in the EU GMP guideline part 15.
Logix Automation GmbH supports its customers in the area of qualification (GAMP 5 Qualification oriented) of machines and plants amongst other things with:
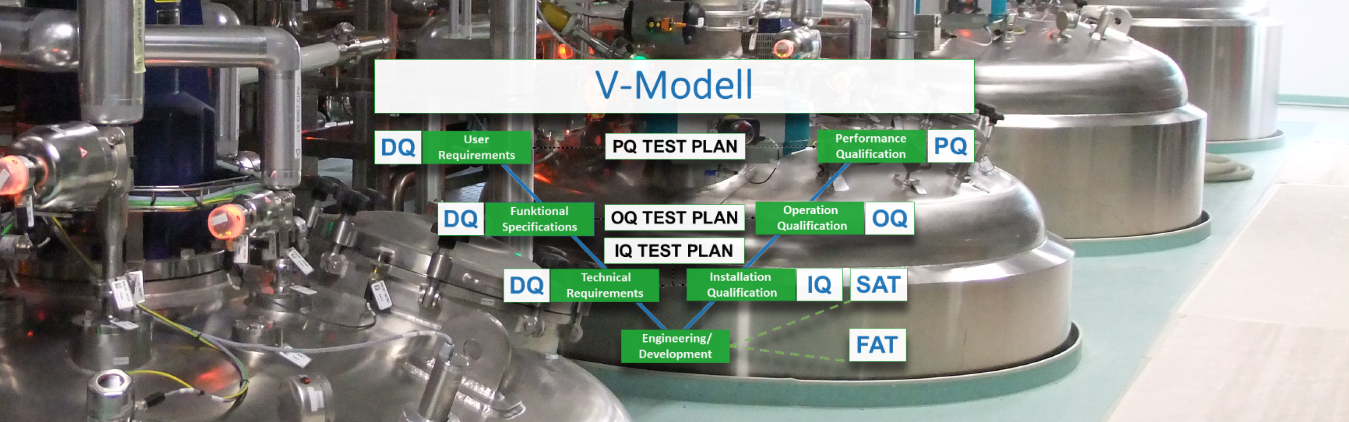
- Installation qualification (IQ): provides documented evidence that devices and systems have been delivered and installed in accordance with GMP-critical requirements and statutory safety regulations.
- Operation qualification (OQ): provides documented evidence that the system functionality defined in accordance with the system specifications is fulfilled in the entire work area within defined limit values. With the approval of the OQ report, a subsequent PQ (production qualification) or a process validation can be started.
- Final qualification report: the entire qualification, its process and results are documented here. When this document is completed, the machine / system to be qualified is also released.
- FDA 21 CFR Part 11: to ensure that electronic records and electronic signatures on computerized systems are credible and reliable.
- GMP risk analysis: a method to evaluate and define critical parameters for the functioning of equipment or process. “EU-GMP Annex 15”. The method of FMEA (Failure Mode and Effect Analysis) is often used.
- Change control: If processes, procedures or the Status of equipment are to be changed in a planned manner, the change must be justified, planned and approved by quality assurance before implementation, and the implementation must then be documented.
- Re-qualification: means ensuring that the system is still in a qualified state after changes AND periodically recurring evaluation of a system within defined time intervals.